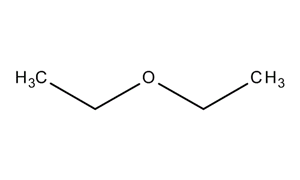
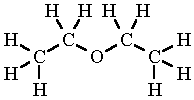Diethyl Ether Structure
It has been used as a recreational drug to cause intoxication.
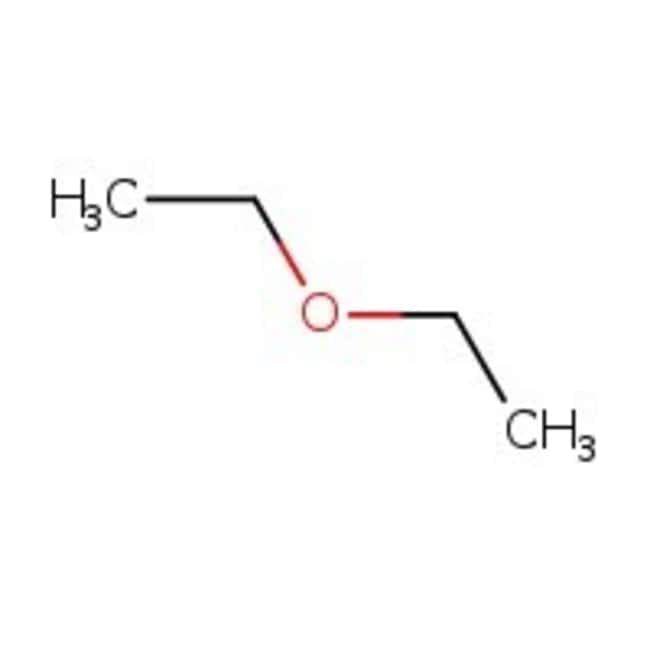
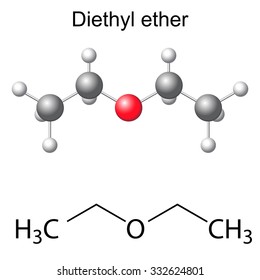
Diethyl ether structure. Molecular formula c 4 h 10 o. It was formerly used as a general anesthetic until non flammable drugs were developed such as halothane. Diethyl ether 2 butanone c8h18o2 cid 19800284 structure chemical names physical and chemical properties classification patents literature biological. Its molecular structure consists of two ethyl groups connected by an oxygen atom as in c2h5oc2h5.
Diethyl ether or simply ether is an organic compound in the ether class with the formula 2o sometimes abbreviated as et 2o. Ethyl ether also known as diethyl ether well known anesthetic generally referred to as simply ether an organic compound belonging to a large group of compounds called ether. It is commonly used as a solvent in laboratories and as a starting fluid for some engines. Monoisotopic mass 74 073166 da.
It is an isomer of butanol. Ethyl alcohol ethyl ether. C 4 h 10 o. A carrier for a hair care product containing polyvinylpyrrolidone was prepared with 77 04 g 70 aq dimethyl ether 14 11 g dimethyl ether 1 09 g co2.
An aerosol mixt contains dimethyl ether 28 97 38 5. Average mass 74 122 da. Ethylbenzene dimethyl ether diethyl ether ethyl bromoacetate 1 4 bromophenoxy 1 ethoxyethane ethyl 2 bromovalerate benzyl 2 bromoacetate ethyl isocyanoacetate ethyl 2 bromopropionate ethyl 2 bromohexanoate ethyl 2 bromoisobutyrate ethyl bromoacetate 1 2 13c2 tert butyl bromoacetate dl ethyl 2 bromobutyrate ethyl 2 bromoheptanoate petroleum ether diethylene glycol monoethyl ether 2 2 dichlorodiethyl ether.