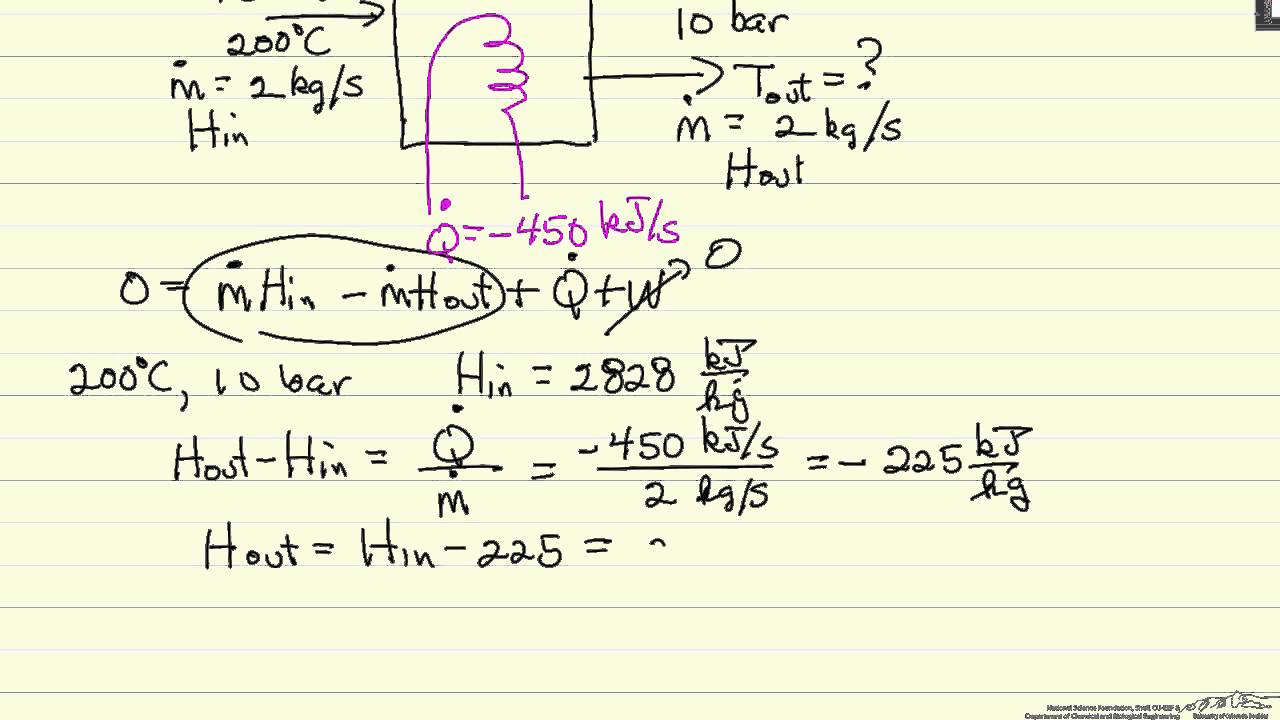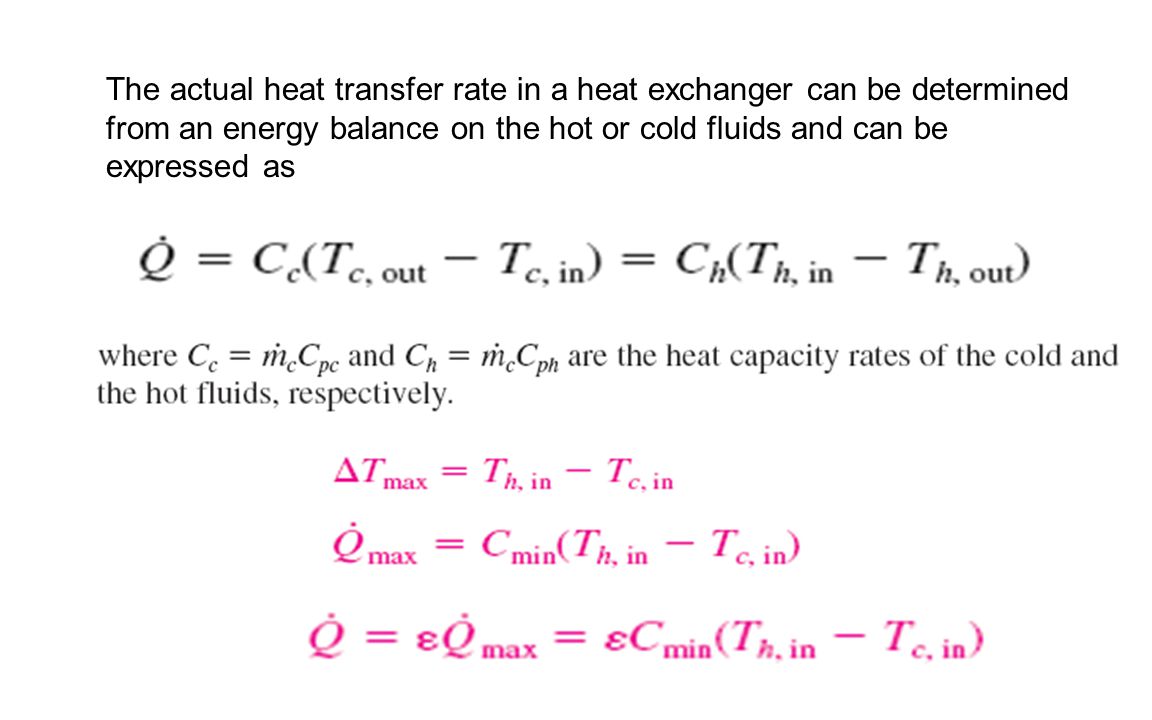Energy Balance Heat Transfer
The mass balance and the temperature variations of a glacier are determined in part by the heat energy received from or lost to the external environment an exchange that takes place almost entirely at the upper surface.
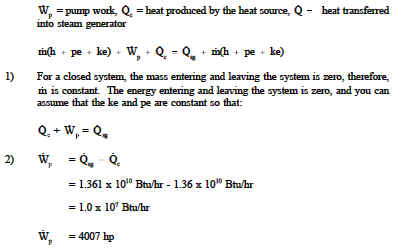
Energy balance heat transfer. The objectives of this paper determine to formulate a global energy balance in a shell and tube heat exchanger and to study the heat losses firstly focuses to study the heat transfer in countercurrent and parallel flow and to measure the temperature profile in a shell and tube heat exchanger. 1 heat gained by the cold side q m c cp c to c ti c. For bphes a larger area. Energy transfer is always from areas of higher to areas of lower energy from warmer to cooler substances.
For hot fluid side of a heat exchanger let heat lost by the hot fluid q m h cp h to h ti h. Heat transfer coefficient k. Specific heat and heat capacity. Heat transfer energy balance heat transfer energy balance josh2008 mechanical op 21 oct 10 11 43.
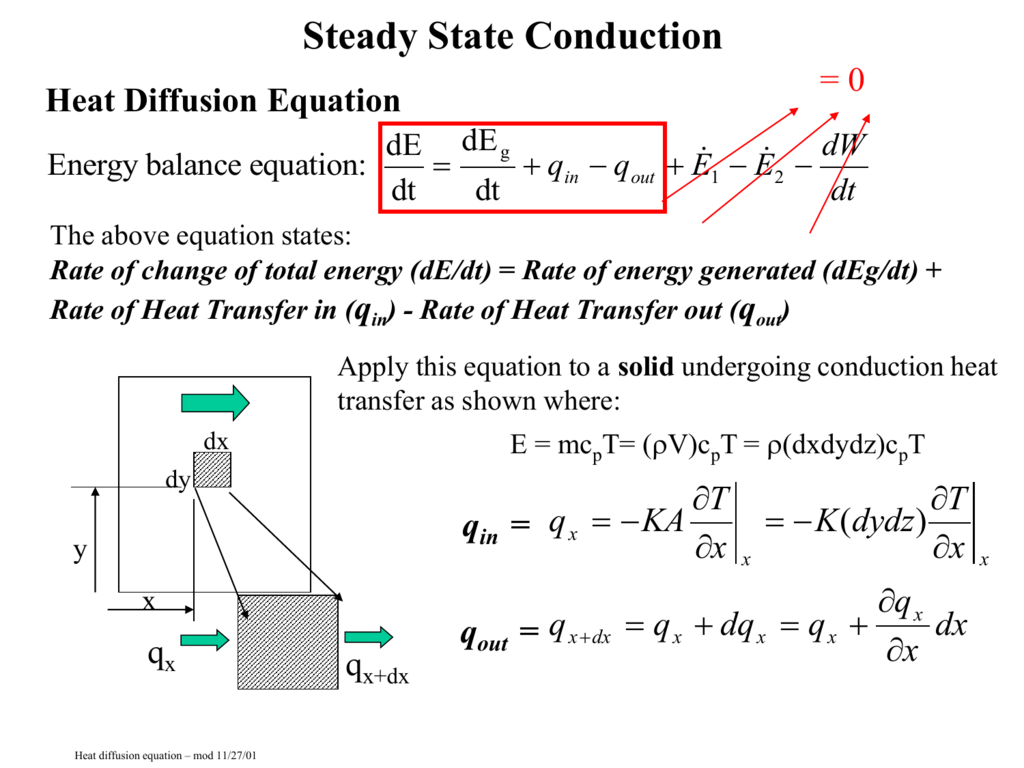
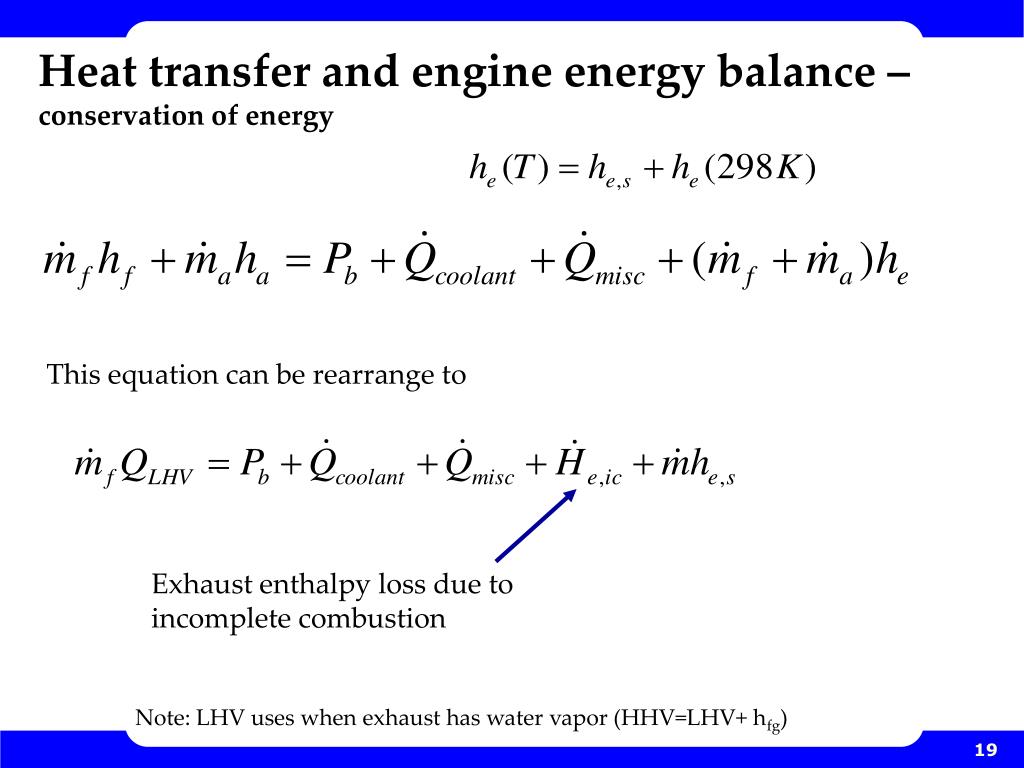
Rate of change of total energy de dt rate of energy generated deg dt rate of heat transfer in qin rate of heat transfer out qout apply this equation to a solid undergoing conduction heat transfer as shown where. Tank 200 gal 0 76 m3 k 235 w mk. That is if q is the heat transferred from the surroundings to the system then q 0 means that net heat is transferred to the system so as to increase the energy of the system. Change in energy over space.
In steady heat transfer the temperature and heat flux at any coordinate point do not change with time both temperature and heat transfer can change with spatial locations but not with time steady energy balance first law of thermodynamics means that heat in plus heat generated equals heat out 8. Forms of heat energy. Heat is lost by outgoing long wavelength radiation turbulent transfer to colder air the heat required for the evaporation sublimation or melting glacier glacier heat or energy balance. 1 5 energy balance area a.
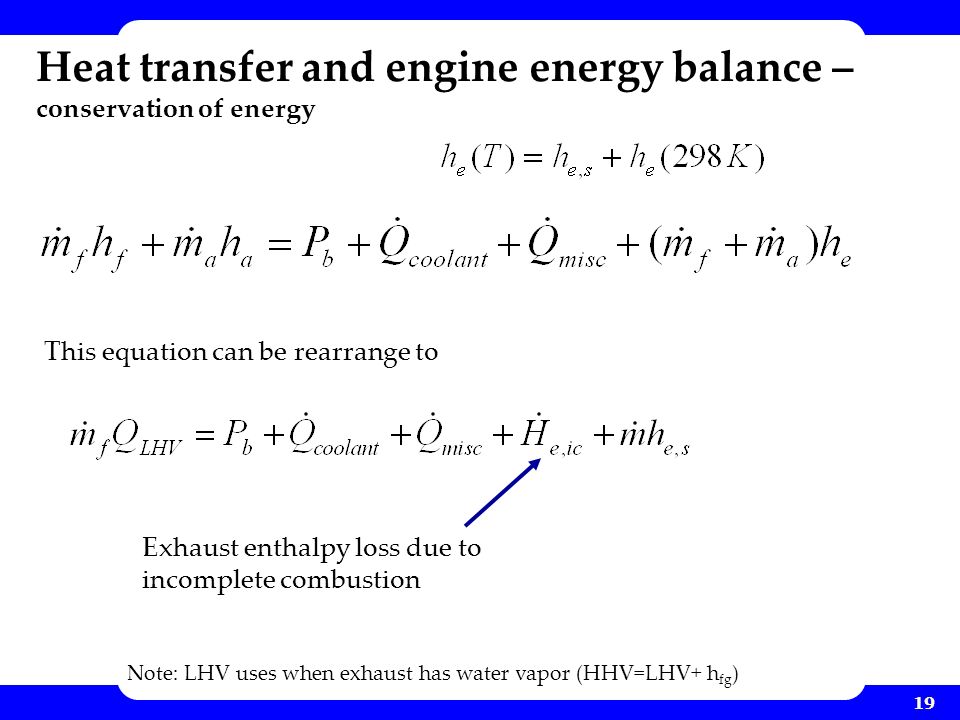
This balance is useful for determining the total heat transfer necessary to accomplish a given temperature change in a system since the internal energy and the enthalpy of any pure substance or mixture can be estimated as a function of temperature given some experimental data using thermodynamics. Numerical value of heat transferred is positive when it is transferred into the system thereby increasing the energy contained in the system. Increasing the area of a heat exchanger implies that more energy can be transferred. If i have a container with hot water coming in and mixed water exiting at the same flow rate what is the energy balance for the system.