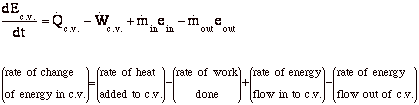Energy Balance Equation Thermodynamics
In its simplest form the energy balance equation is meant to represent what does or at least should happen to the body by looking at the difference between energy intake from food and energy output.
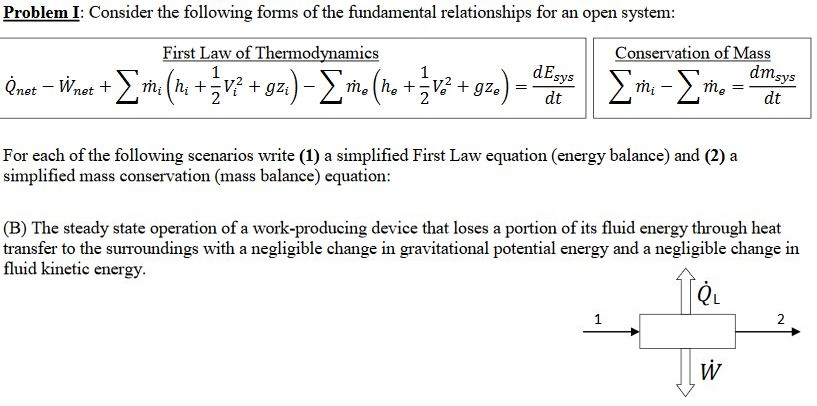
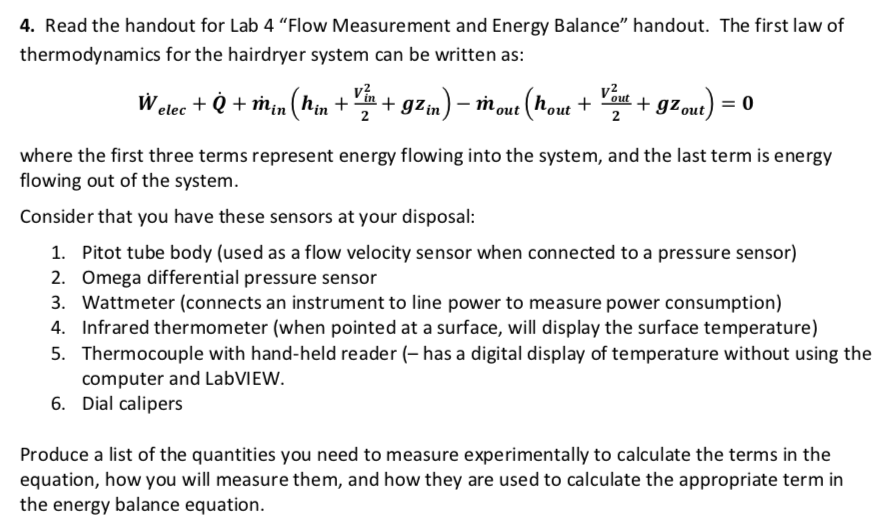
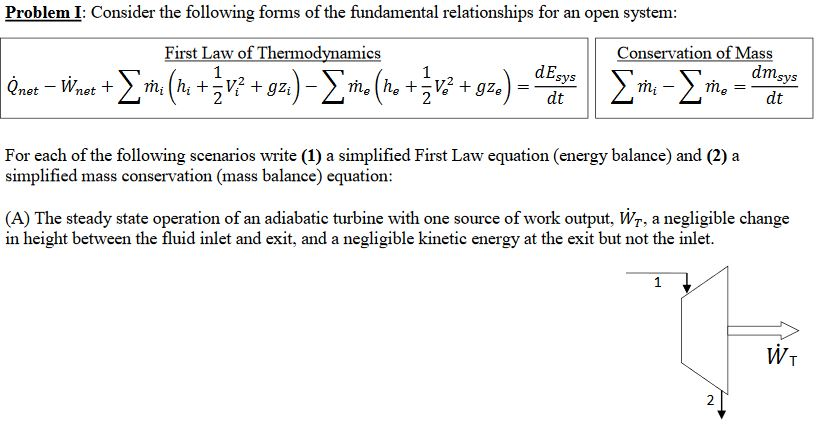
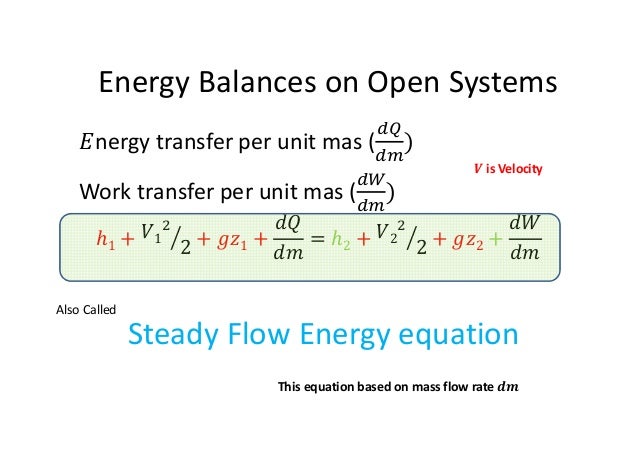
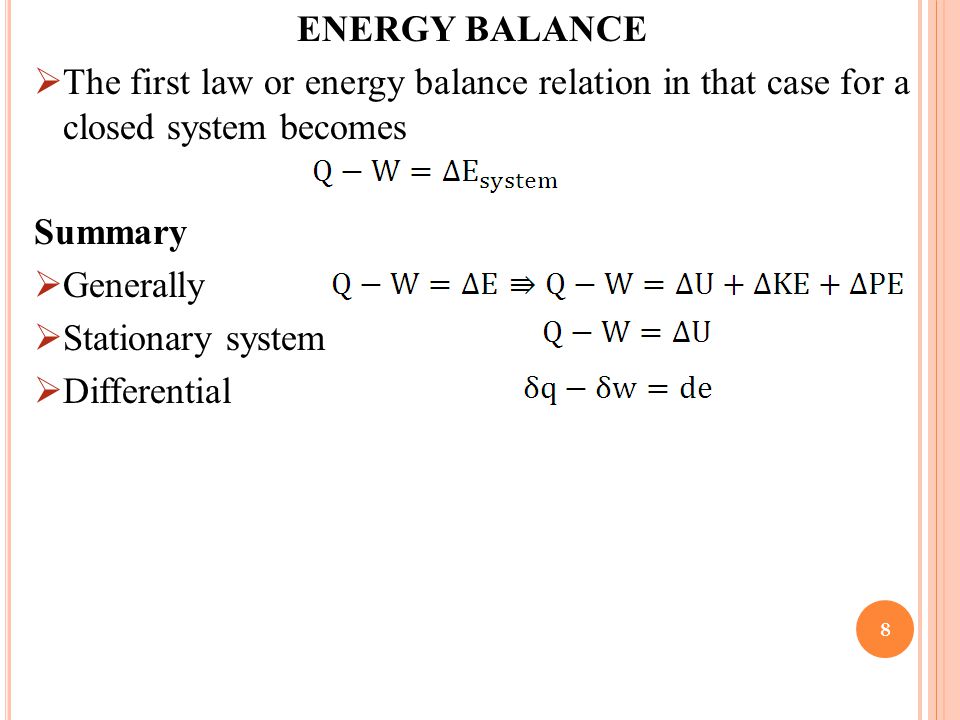
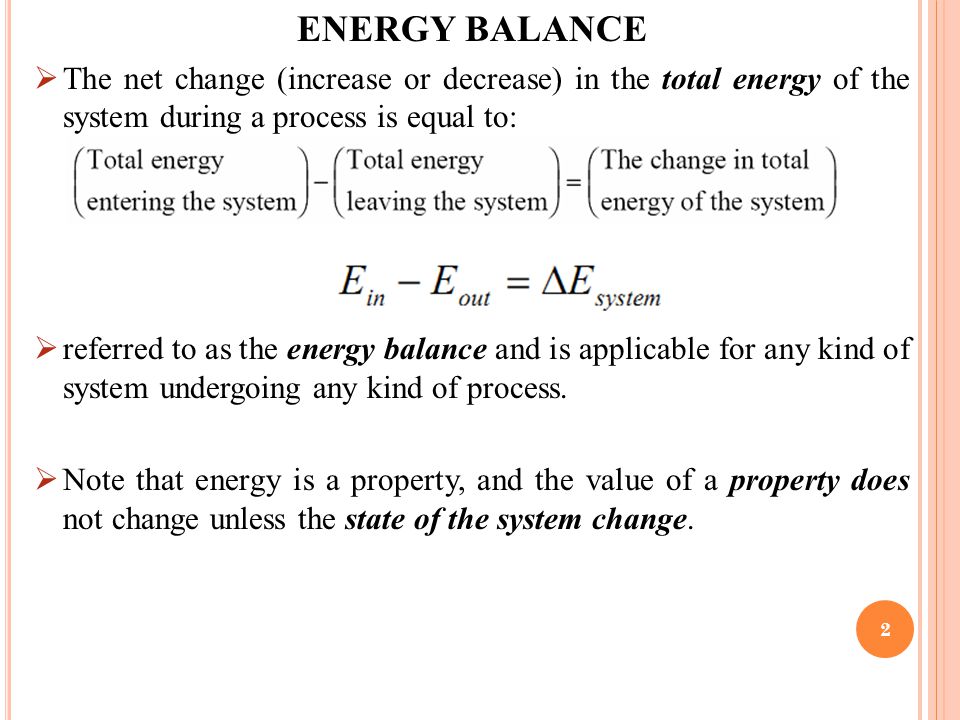
Energy balance equation thermodynamics. The concept which governs the path that a thermodynamic system traces in state space as it goes from one equilibrium state to another is that of entropy. The internal energy has changed from its level at instant 1 to another level at instant 2 after heat is applied. The general energy balance for a process can be expressed in words as. Heat is the energy transfer due to temperature difference.
Therefore the energy balance is. This video is from the free online course. This set of thermodynamics multiple choice questions answers mcqs focuses on energy equation 2. The internal energy u is a thermodynamic property.
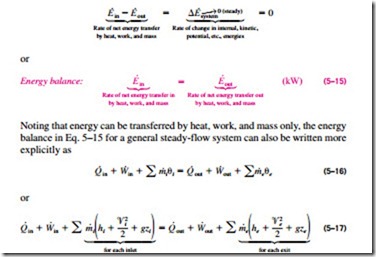
A 490 7 kj b 590 7 kj c 690 7 kj d 790 7 kj view answer. The first law of thermodynamics for the closed system in differential form is du dq dw if the potential and kinetic energy are ignorable. In it s exceedingly simplest form the energy balance equation is this. Above equation of mass balance for a steady flow process is also termed as equation of continuity.
The tank is then cooled to 100 k. Heat engine thermodynamic diagram. A 100l rigid tank contains nitrogen at 3 mpa 900 k. First law of thermodynamics for a stirling engine the first law is simply an energy balance of the system.
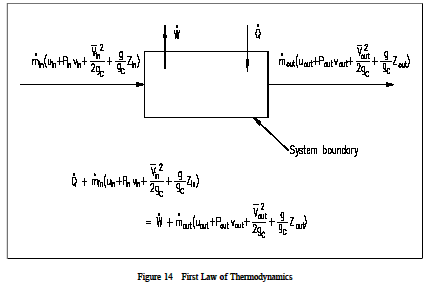
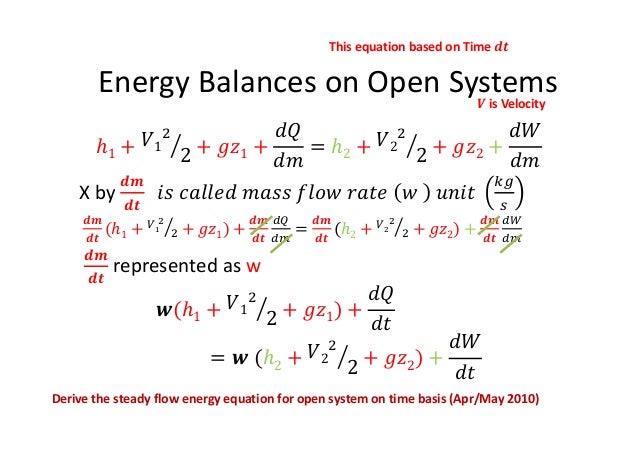
Thermodynamic equations are now used to express the relationships between the state parameters at these different equilibrium state. Where v1 and a1 are the velocity and area of cross section of stream respectively at section 1 1 of control volume system. Comments on thermal efficiency. And work is all the other forms of energy transferring.
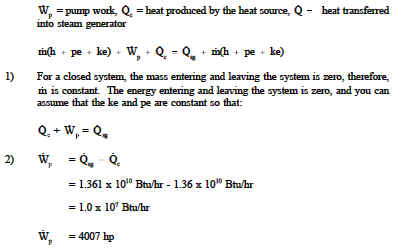
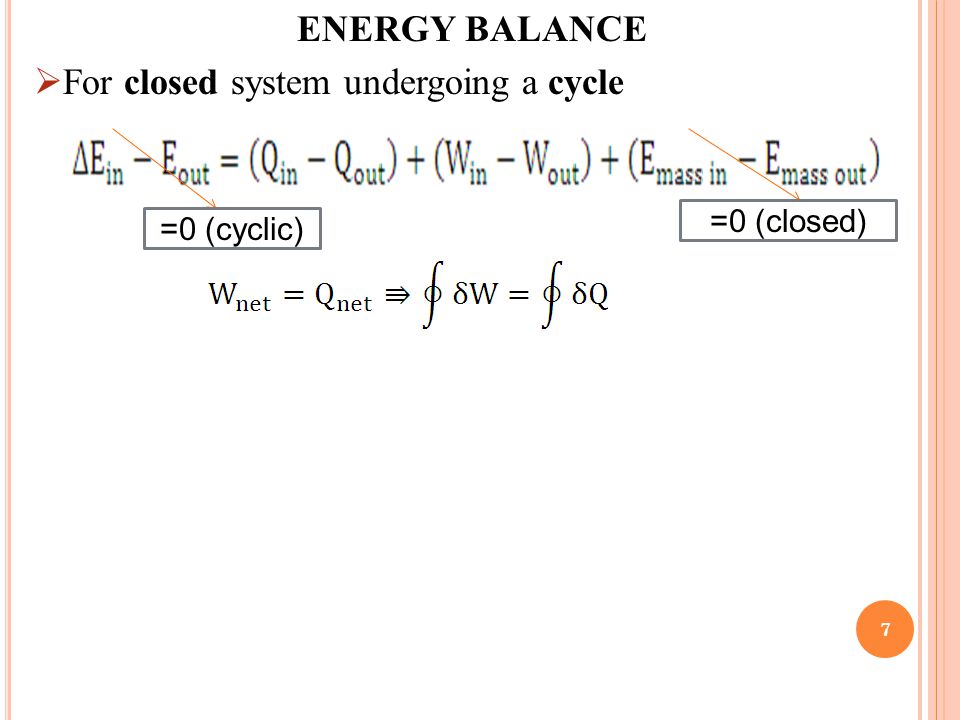
This energy balance is the first law of thermodynamics and always holds. What is the heat transfer for this process. Qq q u u u ce 12 q c is the quantity of heat absorbed by the fluid from the source and q e is the heat loss of the fluid to the environment. Energy in energy out change in body stores.